About targeted protein degradation
Targeted protein degradation
Targeted protein degradation co-opts the cell’s natural disposal systems to remove disease-causing proteins and is applicable to diverse therapeutic areas including oncology, dermatology, immunology, neuroscience and respiratory diseases. As an entirely new approach, it provides hope of treating diseases previously thought to be undruggable.
Degrading rather than inhibiting a target protein offers several advantages such as more efficacious drug response at lower doses, and a more precise intervention with potentially reduced side effects and disease resistance. The first compounds in this class, termed Proteolysis-targeting chimeras (PROTACs), are being trialled as candidate medicines against various cancers and other diseases and progressing through clinical trials.
How PROTAC degraders work
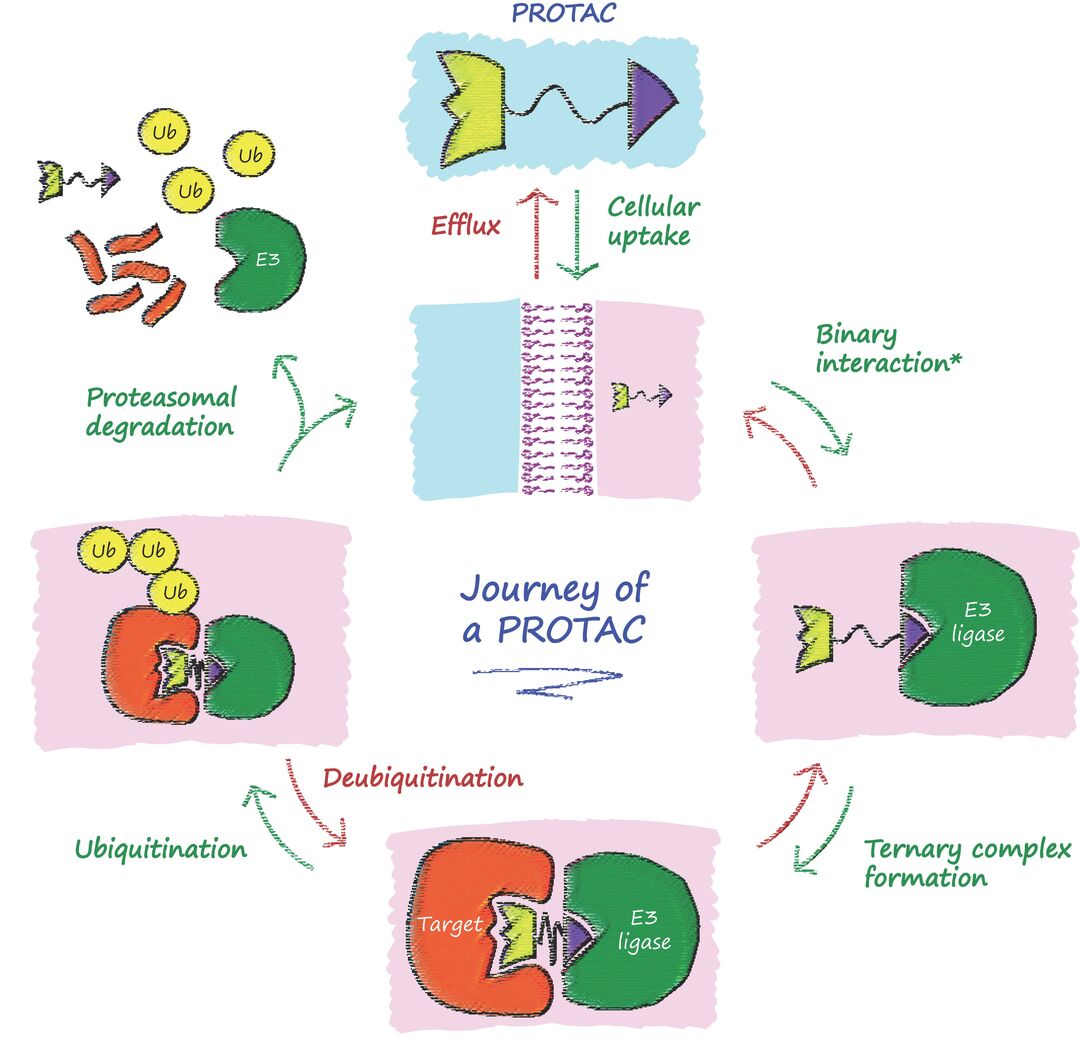
Cartoon depiction each steps along the journey of a PROTAC degrader molecule. Upon treatment, the compound enters into the cell, must bind simultaneously its target protein and E3 ligase, resulting in ubiquitination and ultimately proteasomal degradation of the protein target. Once complete, the PROTAC is free to repeat the cycle. Note: the binary interaction step can occur with either E3 ligase or protein target.
Adapted from Trainor & Ciulli, Biochem (Lond) (2021) bio_2021_148