Professor Grahame HardieFRS FRSE FMedSci
Emeritus Professor
Cell Signalling and Immunology, School of Life Sciences

Contact
Research
The AMP-activated protein kinase (AMPK) cascade was first defined in our laboratory. Almost all energy-consuming reactions in the cell are powered by the high ratio of ATP to ADP, and when energy stress causes this ratio to fall, the AMPK system is switched on. Due to the adenylate kinase reaction, any increase in the ADP:ATP ratio is accompanied by a large rise in AMP, and this is the primary signal that triggers AMPK activation.
AMPK occurs in essentially all eukaryotes as heterotrimers with catalytic alpha and regulatory beta and gamma subunits (See Figure 1 below).
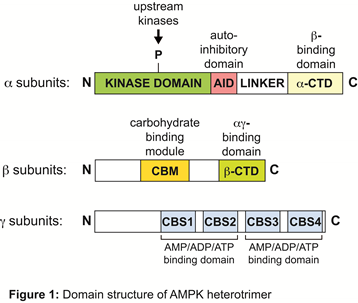
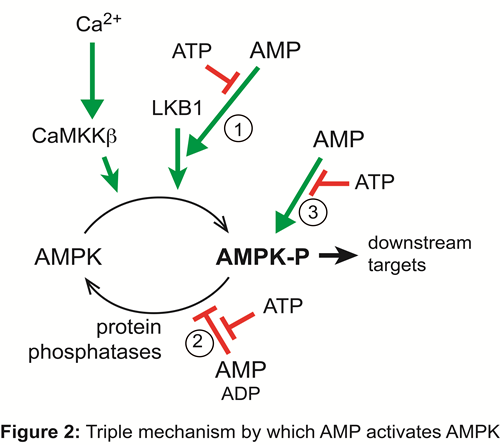
AMP and/or ADP binds to one or more of three sites formed by the CBS repeats on the gamma subunit, causing conformational changes that have three effects, all antagonized by binding of ATP:
- promoting phosphorylation of the alpha subunit at the activating site, Thr172, by the upstream kinase LKB1;
- inhibiting dephosphorylation at Thr172 by protein phosphatases – this effect can be mimicked by ADP;
- allosteric activation of the kinase already phosphorylated on Thr172.
This complex mechanism allows AMPK to act as an ultrasensitive sensor that monitors cellular AMP:ATP and ADP:ATP ratios (Figure 2). AMPK can also be activated by a rise in cellular calcium ions, which switch on the alternative upstream kinase, the calcium/calmodulin-dependent kinase kinase, CaMKK-beta.
Once activated by falling energy status or rising calcium ions, AMPK switches on ATP-producing catabolic pathways, while switching off ATP-consuming processes, including cell growth and proliferation. The AMPK system has also been found to play a key role in modulating energy balance at the whole body level, by mediating effects of hormones such as leptin, adiponectin and ghrelin that regulate food intake and energy expenditure. It is responsible for many of the acute metabolic changes and the longer-term metabolic adaptations of muscle to regular exercise, and may explain the protective effects of regular exercise on the development of obesity and type 2 diabetes. By reducing fat storage in cells, AMPK may also mediate the insulin-sensitizing effects of metformin, an anti-diabetic drug currently prescribed to over 100 million people worldwide.
Our finding in 2003 that LKB1 was the upstream kinase that phosphorylates Thr172 introduced a link between AMPK and cancer. We are currently investigating the role of AMPK in cancer, and whether AMPK activation can explain the apparent protective effects of metformin in development of the disease.
Recently, we discovered that salicylate, a natural product that is the major breakdown product of aspirin, activates AMPK by direct binding to the carbohydrate-binding module on the beta subunit. It is becoming clear that AMPK modulates metabolism in cells of the immune system, and we are interested in the possibility that AMPK may mediate some of the anti-inflammatory effects of aspirin and other salicylate-based drugs.
Awards
| Award | Year |
|---|---|
| National Sciences Prizes awarded since 1990 / Biochemical Society Randle Lecture | 2023 |
| Major Personal Funding Awards / Wellcome Trust Investigator Award | 2016 |
| Major Personal Funding Awards / Wellcome Trust Senior Investigator Award | 2012 |
| National Sciences Prizes awarded since 1990 / The Novartis Medal & Prize of the Biochemical Society | 2010 |
| Honorary Degrees / Doctor Honoris Causa, Medical University of Bialystok, Poland | 2008 |
| International Science Prizes awarded since 1990 / Rolf Luft Award, Karolinska Institute, Sweden | 2008 |
| Fellow of the Royal Society | 2007 |
| Fellow of the Academy of Medical Sciences | 2002 |
| Fellow of the Royal Society of Edinburgh | 1998 |
Stories
News
David Stansfield, who was a Principal Investigator in the Department of Biochemistry at the University of Dundee from 1965 to 2000, revisited the School of Life Sciences on February 5th for the first time since his retirement 25 years ago
Press release
Five University of Dundee researchers have been named on a “who’s who” list of the world’s most influential academics.

News
Professor Grahame Hardie, from the School of Life Sciences, has been awarded the prestigious Sir Philip Randle Lecture in recognition of his record of excellence in biochemistry