Press release
How do cells stay healthy? University researchers unveil key cellular mechanism
Published on 1 March 2024
A critical new understanding of how cells control the production of proteins that dot the surface of cells has been unveiled in a groundbreaking new study

Experts at the University of Dundee’s Medical Research Council Protein Phosphorylation and Ubiquitylation Unit (MRC PPU), working in collaboration with researchers at the University of Leeds, have shed new light on a vital biochemical process called UFMylation, unveiling its crucial role in maintaining cellular health.
The findings have been published in the prestigious journal Nature.
Proteins are the workhorses of the cell, with membrane-embedded and secreted proteins playing particularly important roles. However, the molecular machines that make these proteins (ribosomes) can encounter roadblocks resulting in the formation of aberrant proteins that can be harmful to cellular function.
Quality control systems within cells work to address these issues, ensuring proper protein synthesis and cellular health. Until now, a significant mystery has surrounded how cells manage protein synthesis at the endoplasmic reticulum (ER) membrane, where a large proportion of these proteins are made.
Central to this discovery is UFMylation, a process involving the attachment of a small protein called UFM1 to ribosomes. Researchers at the Kulathu lab at the University of Dundee, working together with Prof. Elton Zeqiraj’s lab at the University of Leeds, have uncovered the intricate workings of this process, revealing a complex known as the UFM1 Ribosome E3 Ligase (UREL) complex. This complex plays a dual role, both modifying ribosomes with UFM1 and then facilitating the release of stalled ribosomes from the ER membrane.
Through innovative techniques, the researchers were able to visualise the UREL complex in action, providing unprecedented insights into its structure and function. Their findings suggest that UFMylation serves as a crucial mechanism for maintaining protein homeostasis within cells, with implications for various pathologies including neurodevelopmental disorders.
Professor Yogesh Kulathu, Principal Investigator, said, "This work not only elucidates the function of UFMylation but also reveals the unique nature of the UREL E3 ligase complex, paving the way for new therapeutic interventions."
This groundbreaking research, funded by organisations including the UKRI Medical Research Council, BBSRC, the Wellcome Trust, the Lister Institute for Preventive Medicine, and the European Research Council (ERC), represents a significant advancement in the understanding of cellular biology. By uncovering the complex mechanisms underlying protein synthesis regulation, this study opens new avenues for research into understanding and treating a range of diseases linked to protein homeostasis.
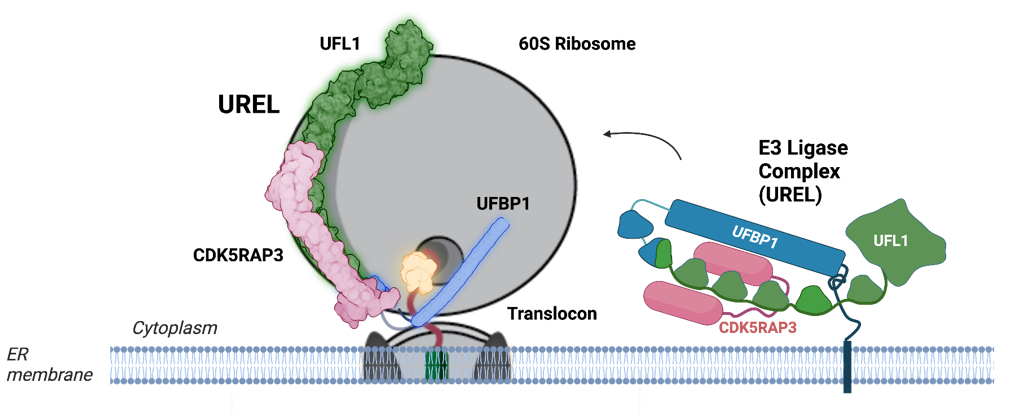
Diagram Description: Schematic cartoon representation illustrating how the UFM1 ligase complex binds to 60S ribosomal subunit. (Figure was generated in Biorender) The ligase complex encircles the ribosome, forming a C-clamp-like architecture. One end of the complex (UFBP1, highlighted in blue) inserts itself into the junction between the ribosome and the translocon, wedging the 60S ribosomal subunit away from the translocon.
The UFM1 E3 ligase recognizes and releases 60S ribosomes from ER translocons Mahklouf L, Peter JJ, Magnussen H, Thakur R, Millrine D, Minshull TC, Harrison G, Varghese J, Lamoliatte F, Foglizzo M, Macartney T, Calabrese AN, Zeqiraj E and Kulathu Y. Nature.
