Factsheet
Approval of international collaborative research projects – guidance for researchers and school research ethics committees
Guidance on approval process for international collaborative research projects for researchers and School Research Ethics Committees (SREC).
Updated on 2 October 2024
1. Introduction
Every year, the University engages in a number of research projects that happen overseas. This includes, but is not limited to: distance students who collect their data in their home countries; staff members in collaboration with international peers; and overseas students who may alternate between collecting data in their home country and in the United Kingdom (UK).
It is recognised that the definition of international collaborative research projects covers a wide variety of projects. For example, it can include small projects where very limited data is collected as well as major international clinical trials. Therefore, depending on the nature of the project, a different pathway may have to be adopted and adjusted accordingly.
Usually, any UK based healthcare related research would be reviewed by an NHS Research Ethics Committee, however, the same approvals process does not apply to healthcare research conducted overseas. As such, those types of projects require to be approved by the relevant SREC in line with this guidance.
For the avoidance of doubt, ‘overseas’ for the purposes of this guidance means outwith the UK.
2. Purpose
This Guidance is intended to cover all research projects, including clinical trials, that take place overseas. It is aimed at: researchers who are planning to carry out research overseas; and School Research Ethics Committees (SRECs) who are considering whether research projects can proceed.
This guidance sets out the procedures that researchers and SRECs should follow in relation to a proposed overseas research project and some of the key principles that should be considered as part of that.
If you have any questions about this guidance please contact The University of Dundee Research Ethics Committee at [email protected].
3. Key principles
The following principles must be considered by the SREC when reviewing all proposed overseas research projects. Researchers should also consider these principles when planning an overseas research project.
Welfare of participants and researchers
The first remit of the ethics committee is to ensure the welfare of any volunteer participants, either at the time of collection of data or later in the research project with issues relating to the use of data, confidentiality and/or treatment of participants. In this sense, members of the SREC who are reviewing a project are encouraged to reflect and raise any issues related to risks involving the participants or volunteers, the researchers and ultimately the University itself.
It is impossible to foresee all eventualities, but reviewers are encouraged to look at aspects that would help safeguard the integrity of volunteer participants, such as free and informed recruitment, with attention to minorities and possible power relationships such as student/teacher and physician/patient. In addition, reviewers should look at what procedures are proposed to mitigate the risk of coercion.
It may be helpful to consider the points set out in the University’s Safeguarding Guidance for Researchers.
Respect relevant regulations in host country
For research projects happening in other countries where the UK jurisdiction does not apply, the rules, regulations and laws of the country in question must be adopted and respected. The University does not have the desire, or indeed would not have the power to impose or recommend different rules abroad.
It is therefore incumbent on the principal investigator, in conjunction with the supervisor for student projects, to ensure that all applicable laws, relevant regulations and guidance are followed and any appropriate licenses are obtained before the collection of data starts.
Seek and obtain ethical approval in country where research is being undertaken
Ethics approval must be sought and obtained in the country where the research activity is to take place. Not all countries will have a structure of ethical approval committees like we do in the UK, therefore, it may be necessary in some cases to seek appropriate permission with the nearest regulating body. That could be, for example, a management council running a hospital or school, a civil authority in charge of the community or any other appropriate protective structure in place.
Ethical standards governing the research should meet minimum requirements in the UK
The minimum requirements will vary from country to country. It is possible that a situation may arise where the University would not want to get involved in carrying out a research project in a particular country. This might happen, for instance, in areas where practices are different to those in the UK such as in the areas of animal welfare and/or attitudes towards minorities and/or vulnerable people. Where possible, the University may still allow that research to proceed but only if the researcher can demonstrate that the research would be acceptable under UK laws and practice.
Types of research project that may require particularly careful attention
The nature of a research project may mean that it will be considered to be a higher risk project. The following project types should be given particularly careful attention:
- Clinical trials (clinical trials abroad are not the jurisdiction of the NHS and the University may be asked to grant approval for collaborative trials happening overseas).
- Research involving minority groups. Groups such as prisoners, children, ethnic, religious, sexual and other minorities must be carefully considered as they may be at risk of inappropriate care and recruitment.
- Research involving animal models.
- Research involving the use of human tissue.
- Research involving genetic manipulation, including IVF and other complex procedures.
- Research involving new drugs.
Additional guidance
Researchers may also find it useful to consider the advice given by the Association of Social Anthropologists of the UK and the Commonwealth and The American Anthropological Association
4. Procedure for researchers
This is the procedure that should be followed by researchers who are planning to carry out a research project overseas. Students must discuss and agree their proposed project with their supervisor before carrying out any of these steps. In some cases you may wish to have a first draft of the application reviewed by your SREC before passing to the host country for their approval. A final application, with evidence of host approval, should then be submitted to and approved by your SREC before you proceed as detailed below.
Step 1. Obtain local ethics approval.
- In the majority of cases, you should first seek and obtain ethical approval in the country where the research project is to take place, as per section 3 above.
Step 2. Submit relevant documents to SREC.
- Once local ethics approval has been granted, you should submit evidence of that approval, such as an approval letter, and any other documentation relevant to your research project to the relevant SREC for review. The best way to do this is by emailing it to the SREC Administrator.
SREC administrator contact details
Step 3. Await sign off from SREC.
- Before you can begin any research activity you must wait until the project has been reviewed by the SREC and you have received confirmation that your research project may proceed.
- If your project involves healthcare research you must also await confirmation of sponsorship from Tayside Medical Science Centre (TASC). You must not start your project before sponsorship is confirmed.
- If you are not granted approval to proceed with your research project and you would like to discuss that decision, please contact the SREC Administrator in the first instance.
5. Procedure for SRECs
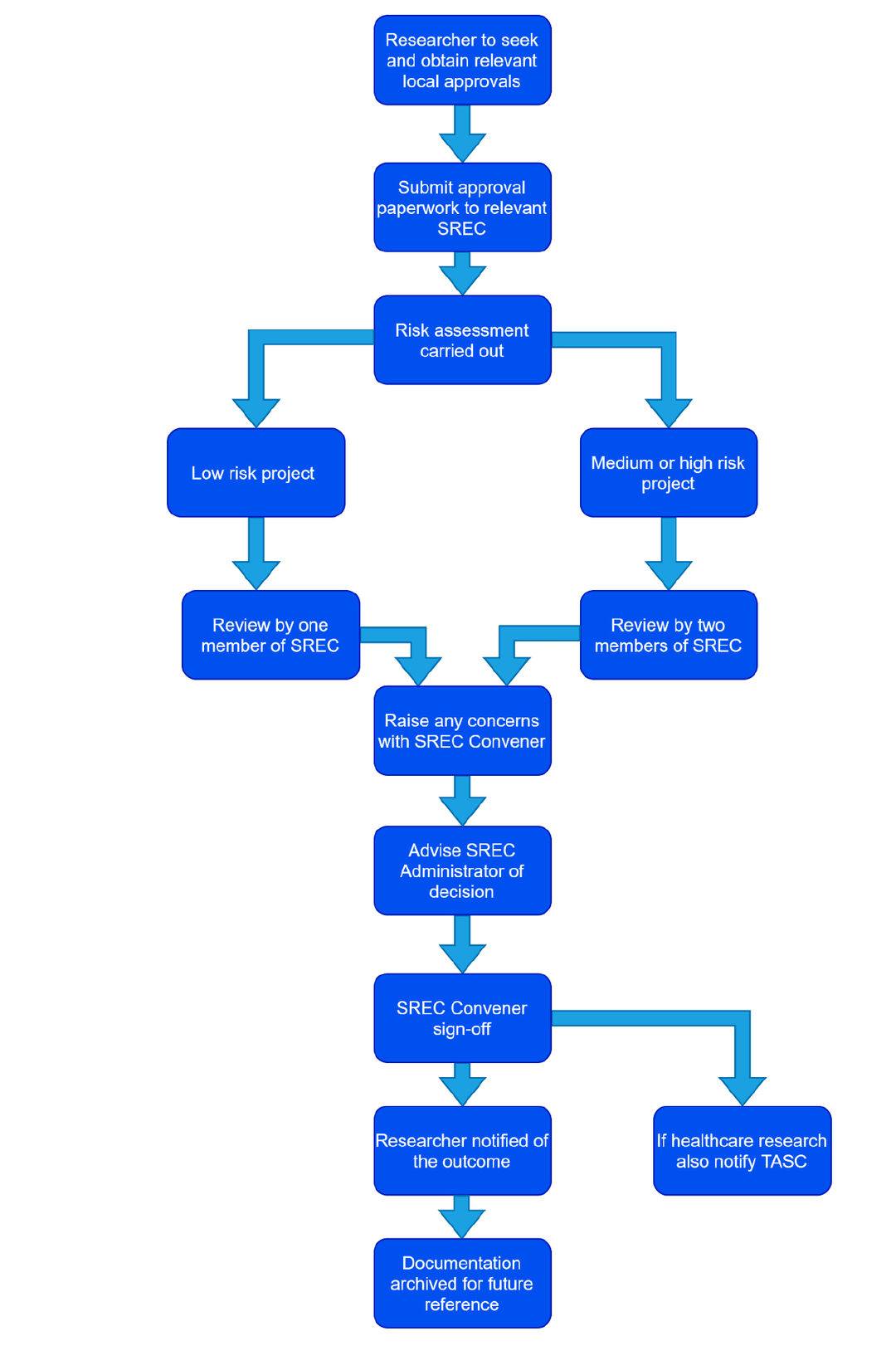
This is the procedure that should be followed when considering whether an overseas research project can proceed or not. A flowchart setting out the different steps can be found at Annex 1.
Step 1. Receipt of relevant documentation
- An application to the SREC should include all documentation relevant to the research project including evidence of any necessary local approvals which should have been sought prior to the application being submitted.
Step 2. Risk assessment
- There may be circumstances where it would be appropriate to seek ethical approval from the University of Dundee first. Researchers are encouraged to discuss with their collaborators at the earliest opportunity.
- A risk assessment must be carried out to determine whether or not the research project is considered to be low, medium or high risk. Checklist 2 should be used to help determine the level of risk.
Step 3. Low risk projects
- If the risk assessment carried out at Step 2 determines that the project is considered to be low risk it can be reviewed by one member of the SREC.
Step 4. Medium and high-risk projects
- If the risk assessment carried out at Step 2 determines that the project is medium or high risk then it should be reviewed by at least two members of the SREC.
Step 5. Raising concerns about the project
- If an SREC reviewer has any concerns about a project they should raise their concerns with the SREC Convener in the first instance. Concerns can be escalated further up to the University Research Ethics Convener (UREC) if the SREC Convener considers that to be appropriate.
- If required, at any time during a review, other members of University staff with particular expertise may be recruited for the purposes of discussing specific cases and considering certain issues. For example, a project may involve technical, financial or data controlling issues and it may be helpful to seek advice from colleagues in UoDIT, Finance or Information Governance.
- Only once any concerns raised have been either resolved or appropriately mitigated should the SREC reviewer(s) make a decision about whether it is suitable for the research project to proceed.
Step 6. Decision, sign-off and notification of outcome
- SREC members reviewing a research project must determine, taking into account the principles set out in section 3 above and any other relevant information, whether the project would be compliant with UK standards. If they are satisfied that the project is compliant and that it is appropriate for the University to be involved in such a project, then they may grant approval for the project to proceed.
- Once a review has been completed the reviewer(s) should notify the SREC Administrator of their decision. The SREC Administrator will send details of the decision and all relevant information to the SREC Convener for final sign-off.
- If the SREC Convener agrees with the decision they should confirm that to the SREC Administrator who shall notify the researcher by confirming to them whether their project may proceed or not.
- If the SREC reviewer(s) and the SREC Convener do not agree on what the outcome should be, the matter should be escalated to the Convener of UREC.
- If the project involves healthcare research then the final SREC decision, together with confirmation of local ethical approval, should be sent to TASC Research Governance - [email protected] – so that they can confirm sponsorship. Projects must not start before sponsorship is confirmed by TASC.
Step 7. Archiving of documentation
Once all relevant parties have been notified of the decision, the application, record of the SREC decision, and all other relevant documentation should be archived in the SREC’s files for future reference.
Annex 1 – Flowchart
The University of Dundee Research Ethics Committee
[email protected]